Divergent reactions of oxindoles with amino alcohols via the borrowing hydrogen process: oxindole ring opening vs. C3 alkylation†
Abstract
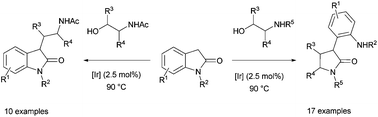
Oxindoles display a diverse pattern of reactivity upon reaction with different classes of amino alcohols via the borrowing hydrogen process. Whereas the reaction with N-acetyl amino alcohols leads to C3 alkylation, the reaction with N-alkyl amino alcohols results in the formation of synthetically useful α-aryl-lactams via a remarkably facile oxindole ring-opening reaction. Reactions occurred with quantitative conversion and selectivity under relatively mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...