Abstract
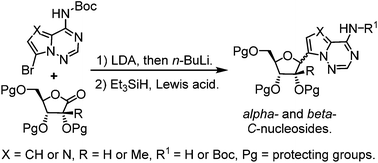
C-Nucleosides constitute a valuable class of compounds in biological and medicinal chemistry studies. We report herein a new and efficient synthesis of both alpha- and beta-C-nucleosides with high anomeric selectivity from N6-Boc protected purine analogues. The synthetic approach features a carefully designed lithiation and silane reduction sequence. The anomeric stereochemistry outcome is dictated by the protecting group of sugar lactones. Computational studies suggest that previously neglected interactions between partially positively-charged silane and the substitutions on a sugar moiety play important roles in the anomeric selectivity of silane-mediated C-nucleoside synthesis.


 Please wait while we load your content...
Please wait while we load your content...