An efficient approach to 4-chloro quinolines via TMSCl-mediated cascade cyclization of ortho-propynol phenyl azides†
Abstract
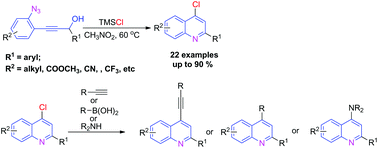
A novel and efficient strategy for the synthesis of 4-chloro quinolines via the TMSCl-mediated cascade cyclization of easily prepared ortho-propynol phenyl azides is developed. This reaction proceeds smoothly in moderate to excellent yields with a wide range of substrate compatibility. TMSCl acted not only as a promoter, but also as a chloro source in this transformation. Moreover, the obtained 4-chloro quinolines could be further derivated in an array of palladium-catalyzed coupling and nucleophilic substitution reactions to construct potential biologically active and pharmaceutical molecules.


 Please wait while we load your content...
Please wait while we load your content...