Photoredox radical C–H oxygenation of aromatics with aroyloxylutidinium salts†
Abstract
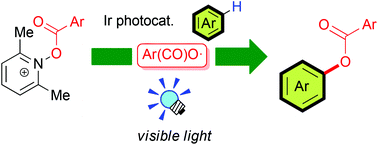
The photoredox-catalyzed aroyloxylation of aromatic C–H bonds with N-aroyloxylutidinium salts giving phenol derivatives has been developed. The present peroxide-free photocatalytic system is a convenient protocol for the generation of O-centered aroyloxy radicals under mild reaction conditions. In particular, N-3,5-bis(trifluoromethyl)phenylcarbonyloxylutidinium salt serves as an efficient aroyloxylating reagent by the action of Ir photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...