Highly diastereoselective access to polyfunctionalized 1,3-oxazines promoted by Brønsted/Lewis acids†
Abstract
The Brønsted/Lewis acid catalyzed reaction of pyrrolidone, piperidone and isoindolinone derivatives tethered to α,β-unsaturated ketones or aldehydes afforded exclusively polyfunctionalized 1,3-oxazines with high diastereoselectivities and in high yields.
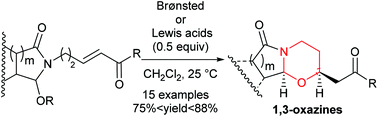


 Please wait while we load your content...
Please wait while we load your content...