Radical-mediated synthesis of γ-lactones by copper-catalyzed intermolecular carboesterification of alkenes with α-carbonyl alkyl bromides and H2O†
Abstract
A novel copper-catalyzed intermolecular carboesterification strategy of alkenes with α-carbonyl alkyl bromides is described. This reaction represents a facile and straightforward access to polysubstituted γ-lactone skeletons, including γ-lactones having ortho-substituted groups on the carbonyl group, in moderate to good yields via a radical strategy, which has good functional group tolerance.
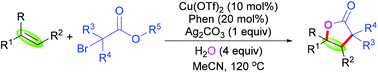


 Please wait while we load your content...
Please wait while we load your content...