Harnessing the potential diversity of resinic diterpenes through visible light-induced sensitized oxygenation coupled to Kornblum–DeLaMare and Hock reactions†
Abstract
A biomimetic procedure for the late-stage functionalization of various resinic acids is reported, implementing photooxygenation by singlet oxygen, using visible light and meso-tetraphenylporphyrin, combined to the Kornblum–DeLaMare reaction or the Hock rearrangement. In addition to giving access to several natural and unnatural diterpenes by the simplest manner, these transformations furnish biosynthetic clues expandable to other natural product families.
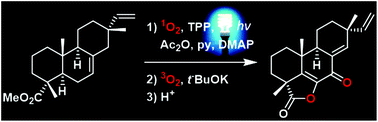
- This article is part of the themed collections: Synthetic Approaches to Natural Products via Catalytic Processes and Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...