Elaborating the excited-state proton transfer behaviors for novel 3H-MC and P2H-CH
Abstract
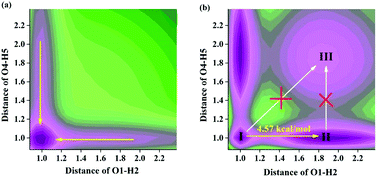
In the present work, we theoretically study and elaborate the excited state proton transfer (ESPT) behavior of two novel polyimides (3H-MC and P2H-CH) reported in the previous work [K. Kanosue, T. Shimosaka, J. Wakita and S. Ando, Macromolecules, 2015, 48, 1777; K. Kanosue, R. Augulis, D. Peckus, R. Karpicz, T. Tamulevicius, S. Tamulevicius, V. Gubinas and S. Ando, Macromolecules, 2016, 49, 1848]. Using density functional theory (DFT) and time-dependent DFT (TDDFT) methods with electrostatic potential surfaces and the reduced density gradient (RDG), we affirm that an intramolecular hydrogen bond should be formed in the ground state for these two systems. Comparing bond lengths, bond angles and the corresponding infrared (IR) vibrations of intramolecular hydrogen bonds for 3H-MC and P2H-CH, we clarify that hydrogen bonds should be strengthened in the S1 state, which provides the possibility for the excited state intramolecular proton transfer (ESIPT) reaction. Analyses of frontier molecular orbitals (MOs) theory prove that the ESIPT process could be facilitated by charge transfer upon excitation. Based on constructing potential energy surfaces (PESs), we provide the excited state dynamical overall perspective about ESIPT reactions for both 3H-MC and P2H-CH. We not only clarify the ESPT mechanism of these two systems, but also make contributions for the applications of such kinds of systems in the future.

 Please wait while we load your content...
Please wait while we load your content...