A modular approach to highly functionalized 3-sulfonylfurans via conjugate addition of 3-cyclopropylideneprop-2-en-1-ones with sodium sulfinates and sequential 5-endo-trig iodocyclization†
Abstract
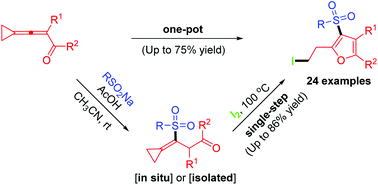
A highly efficient conjugate addition and 5-endo-trig cyclization reaction of 3-cyclopropylideneprop-2-en-1-ones with sodium sulfinates in the presence of iodine is described. A wide variety of densely functionalized 3-sulfonylfurans are furnished by single-step and one-pot methods, respectively. The results of such postcyclization modifications are also presented.


 Please wait while we load your content...
Please wait while we load your content...