Iron-catalyzed boration of allylic esters: an efficient approach to allylic boronates†
Abstract
An efficient approach to allylic boronates via iron-catalyzed boration of allylic esters is described. The reactions of allylic esters and bis(pinacolato)diboron (B2pin2) proceed smoothly catalyzed by FeCl2/PPh3. Thus, a wide range of allylic boronates with broad functional-group compatibility are obtained.
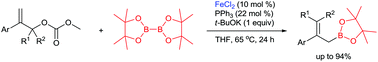


 Please wait while we load your content...
Please wait while we load your content...