Highly enantioselective nitro-Mannich reaction of ketimines under phase-transfer catalysis†
Abstract
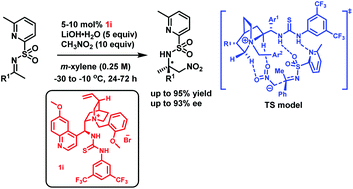
The enantioselective nitro-Mannich reaction of unactivated ketone-derived imines (ketimines) has long been a challenge. With the introduction of the protecting group 6-methyl-2-pyridylsulfonyl to the ketimines, this challenging reaction was performed successfully by using a novel bifunctional quaternary ammonium salt bearing a thiourea group derived from quinine, and when relatively small quantities of nitroalkanes were used, the corresponding products were obtained in good to high yields with high enantioselectivities (up to 93% ee). Density functional theory (DFT) calculations are also performed to give the possible transition-state model.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...