Silver(i) catalyzed intramolecular cyclization of N-(2-(alk-1-yn-1-yl))-1H-tetrazoles leading to the formation of N-cyano-2-substituted indoles under ambient conditions†
Abstract
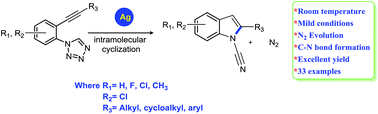
A facile silver(I) catalyzed intramolecular cyclization reaction of alkynyl tetrazoles to form N-cyano-2-substituted indoles has been investigated. This unique cyclization involves the formation of a C–N bond during the intramolecular cyclization of N-(2-(alk-1-yn-1-yl))-1H-tetrazoles in the presence of silver(I) acetate. Good to excellent yields were obtained using low catalyst loadings, ∼5 mol%, under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...