A facile approach for the trifluoromethylthiolation of methylenecyclopropanes†
Abstract
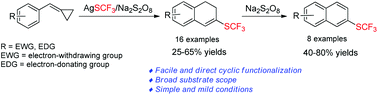
A facile approach for the trifluoromethylthiolation of methylenecyclopropanes (MCPs) has been developed by using AgSCF3/Na2S2O8 as a trifluoromethylthiolation source (SCF3) to give trifluoromethylthiolated 1,2-dihydro-naphthalene derivatives in moderate to good yields, and the reaction has been proven to go through a radical-type pathway. The products can easily be aromatized upon oxidation, offering a new method for the construction of trifluoromethylthiolated naphthalenes.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...