A highly efficient one-pot trifluoromethylation/cyclization reaction of electron-deficient 1,3-conjugated enynes: modular access to trifluoromethylated furans and 2,3-dihydrofurans†
Abstract
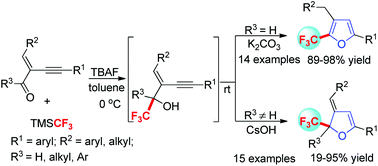
A highly efficient one-pot trifluoromethylation/cyclization reaction of conjugated enyne aldehydes and ketones was developed, which provides modular access to highly substituted trifluoromethylated furans and 2,3-dihydrofurans. This method has the advantages of mild reaction conditions, wide functional group tolerance, high yields and being transition metal free.

 Please wait while we load your content...
Please wait while we load your content...