Efficient construction of the A/C/D tricyclic skeleton of palhinine A†
Abstract
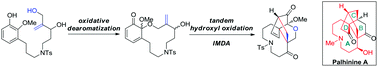
An efficient approach for the synthesis of the 9/6/6 tricyclic structure of Lycopodium alkaloid palhinine A has been accomplished. The developed synthetic route features oxidative dearomatization and tandem hydroxyl oxidation/intramolecular Diels–Alder (IMDA) reactions to assemble the A/C/D tricyclic ring system. Most importantly, the protocol can undergo ring constriction to rapidly construct the highly strained nine-membered azonane ring of palhinine A.

 Please wait while we load your content...
Please wait while we load your content...