Cp*Co(iii)-catalyzed vinylic C–H bond activation under mild conditions: expedient pyrrole synthesis via (3 + 2) annulation of enamides and alkynes†
Abstract
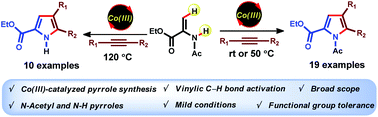
Cobalt(III)-catalyzed (3 + 2) oxidative annulation of enamides and alkynes for the synthesis of pyrroles has been developed under exceedingly mild conditions. The reaction works well with various internal alkynes with broad scope and functional group tolerance. In most cases, the reaction proceeds at room temperature leading to N-acetyl pyrroles in excellent yields. Synthetically useful N-H pyrroles can also be obtained at elevated temperature.

 Please wait while we load your content...
Please wait while we load your content...