A palladium-catalyzed tandem reaction of 2-alkynylbenzenesulfonamides with 2-(2-bromoarylidene)cyclobutanones†
Abstract
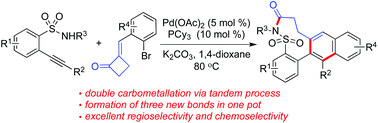
The core of benzo[f][1,2]thiazonin-5(4H)-one 3,3-dioxide is efficiently assembled through a palladium-catalyzed tandem reaction of 2-alkynylbenzenesulfonamides with 2-(2-bromobenzylidene)cyclobutanones. A range of polycycles with a nine-membered sultam ring are generated in moderate to good yields. During the transformation, three new bonds are formed via a tandem process with the construction of a nine-membered sultam ring. Double carbometallation is believed to be involved with the ring-opening of cyclobutanone.

 Please wait while we load your content...
Please wait while we load your content...