Metal-free oxidative hydrophosphinylation of 1,7-enynes†
Abstract
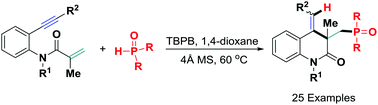
A new metal-free oxidative hydrophosphinylation of 1,7-enynes for forming polyfunctionalized 3,4-dihydroquinolin-2(1H)-ones has been realized using readily accessible diarylphosphine oxide and TBPB as an oxidant. The reaction pathway involves an in situ-generated P-centered radical-triggered α,β-conjugate addition/6-exo-dig cyclization/H-abstraction sequence, providing a direct and promising protocol for the formation of C–P, C–C and C–H bonds and rapid construction of complex heterocyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...