Dearomative C–C and C–N bond cleavage of 2-arylindoles: transition-metal-free access to 2-aminoarylphenones†
Abstract
A transition-metal-free conversion of 2-arylindoles to 2-aminoarylphenones, using environmentally benign O2 as the sole oxidant, has been developed. This novel oxidative dearomatization process involves cleavage of both C–C and C–N bonds followed by new C–C and C–O bond formation. The C2 carbon of an indole scaffold was released in the form of CO2.
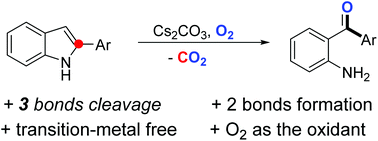

 Please wait while we load your content...
Please wait while we load your content...