Copper-catalyzed tandem A3-coupling–isomerization–hydrolysis reactions of aldehydes and terminal alkynes leading to chalcones†
Abstract
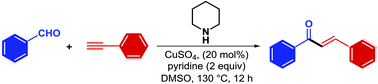
Catalyzed by a simple copper salt, the reaction of an aryl aldehyde and a terminal alkyne in the presence of piperidine delivers the valuable chalcone products, which were rarely found in previous A3 coupling reactions. This reaction can be applied to a wide range of substrates. Mechanistic studies indicate that this reaction experiences a tandem process containing A3 coupling, copper assisted isomerization of propadienamine to allenylamine and subsequent hydrolysis.

 Please wait while we load your content...
Please wait while we load your content...