The development of carbene-stabilized N–O radical coupling strategy in metal-free regioselective C–H azidation of quinoline N-oxides†
Abstract
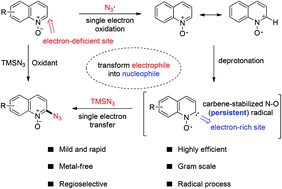
Although radical oxidative coupling provides a powerful C–H functionalization tool, the selective coupling of an electrophilic radical with a heterocyclic electron-deficient C2 site is still a seemingly impossible challenge. In this work, an efficient oxidative C2–H azidation of quinoline N-oxides with TMSN3 has been developed, proceeding at room temperature within 10 min. Based on control experiments and detailed EPR studies, the reaction appears to involve a novel carbene-stabilized N–O radical intermediate, which undergoes a single electron transfer to TMSN3 to give the C2-azidation product. Moreover, this procedure could be smoothly scaled up to gram-synthesis and the products could be converted into other functional heterocycles.

 Please wait while we load your content...
Please wait while we load your content...