Synthesis of Passerini adducts from aldehydes and isocyanides under the auxiliary of water†
Abstract
An efficient protocol for the synthesis of the Passerini adducts α-acyloxycarboxamides from aldehydes, isocyanides and water in a molecular ratio of 3 : 1 : 3 was described. The possible mechanism for the reaction was discussed by employing cross condensation and H218O isotope labeling experiments. This method offers a straightforward access to α-acyloxycarboxamides bearing two identical functional groups in high yields under mild conditions.
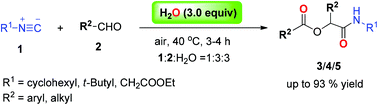

 Please wait while we load your content...
Please wait while we load your content...