Aza-Knoevenagel-type condensation of secondary amides: direct access to N-monosubstituted β,β-difunctionalized enamines†‡
Abstract
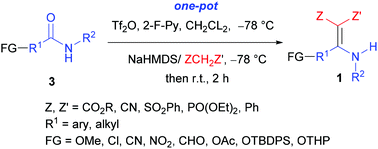
An efficient approach to N-monosubstituted β,β-difunctionalized enamines, a class of versatile building blocks for the synthesis of bioactive compounds, is reported. The method is based on the triflic anhydride-mediated direct aza-Knoevenagel-type condensation of secondary amides with active methylene compounds. The reaction showed good chemoselectivity and functional group tolerance. A number of title compounds have been synthesized in good to excellent yields in one pot from readily available starting materials.

 Please wait while we load your content...
Please wait while we load your content...