Enantioselective nickel-catalyzed alkylative alkyne–aldehyde cross-couplings†
Abstract
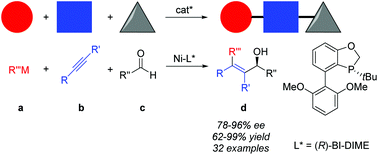
An efficient asymmetric nickel-catalyzed alkylative alkyne–aldehyde cross-coupling is developed by employing a P-chiral monophosphorus ligand BI-DIME, allowing rapid access to a series of chiral tetra-substituted olefinic allylic alcohols in high yields and good to excellent ees. The three-component reactions enjoy excellent regio- and enantioselectivities, and a broad substrate scope from readily available starting materials.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...