CuBr2-catalyzed enantioselective routes to highly functionalized and naturally occurring allenes†
Abstract
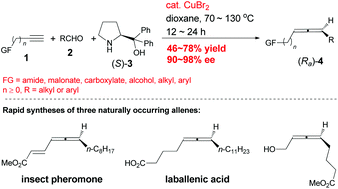
Here we show the CuBr2-catalyzed approach for highly enantioselective synthesis (90–98% ee) of allenes bearing a very broad array of unmasked synthetically attractive functionalities from aldehydes and terminal alkynyl bearing reactive functionalities with the absolute configuration controlled by applying readily available (R)- or (S)-α,α-diphenylprolinol. Following this protocol, the highly enantioselective synthesis of some naturally occurring allenes loaded with reactive functionalities becomes simple: a terminal alkyne plus an aldehyde. In comparison, they were reported to be synthesized either from similar level generic chemicals with much more steps or in lower ees.

 Please wait while we load your content...
Please wait while we load your content...