Cu-catalyzed sp3 C–H bond oxidative functionalization of alkylazaarenes and substituted ethanones: an efficient approach to isoxazoline derivatives†
Abstract
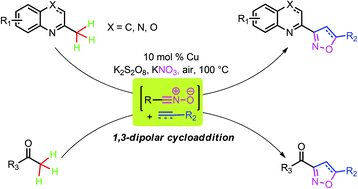
Cu-catalyzed sp3 C–H bond oxidative functionalization of alkylazaarenes and substituted ethanones to different kinds of isoxazoline derivatives by 1,3-dipolar cycloaddition is reported. Cheap sources of nitro, commercially available substrates, as well as a variety of alkenes (alkynes) are applied in this transformation.

 Please wait while we load your content...
Please wait while we load your content...