New building blocks for iminosugars: a concise synthesis of polyhydroxylated N-alkoxypiperidines through an intramolecular azepine ring contraction†
Abstract
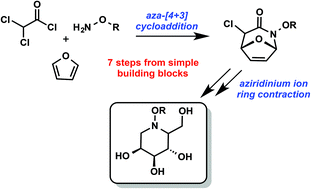
Polyhydroxylated piperidines are a functionally rich class of biologically active molecules that have broad therapeutic potential. Recently developed aza-[4 + 3] cycloadditions of putative aza-oxyallylic cations provide heterocyclic scaffolds that enabled a concise synthesis of polyhydoxylated piperidines. Chemoselective amide reduction and reductive hemiaminal ring opening was achieved in one pot by the action of aluminium hydride generated in situ via aluminium chloride and lithium aluminium hydride. Aziridinium ion mediated ring contraction and chloride displacement was triggered by silver acetate, followed by simple acetate hydrolysis using potassium carbonate to give four tetrahydropyridine diols. Olefin oxidation by osmium tetroxide installed the final hydroxyl groups, which yielded four novel polyhydroxylated N-alkoxypiperidines in good overall yield and high diastereoselectivity.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2015 and Celebrating the 80th Birthday of Professor Ei-ichi Negishi


 Please wait while we load your content...
Please wait while we load your content...