Asymmetric synthesis of poly-substituted spirocyclohexane oxindole via a squaramide catalyzed cascade Michael–Michael–aldol sequence†
Abstract
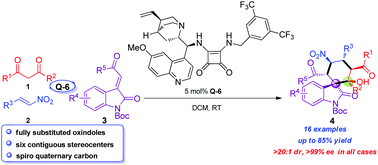
A squaramide-catalyzed Michael–Michael–aldol cascade sequence of three readily accessible substrates (1,3-dicarbonyl compound, nitroalkene and methyleneindolinone) was developed. The reactions led to a series of enantioenriched spirocyclohexane oxindoles bearing six contiguous stereocenters in good yields (up to 85%) and with excellent stereoselectivities (>20 : 1 dr, >99% ee).

 Please wait while we load your content...
Please wait while we load your content...