Synthesis of monolateral and bilateral sulfur-heterocycle fused naphthalene diimides (NDIs) from monobromo and dibromo NDIs†
Abstract
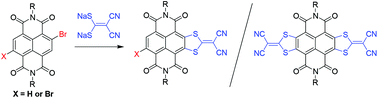
Monolateral and bilateral sulfur-heterocycle fused naphthalene diimides (NDIs) are successfully synthesized from monobromo and dibromo NDIs via nucleophilic aromatic substitution reactions (SNAr) and the subsequent oxidative aromatization processes. The bromo-substituted monolateral fused NDI can be further functionalized with other aromatic or heteroaromatic rings to construct new electron-deficient π-functional materials with fine-tuned molecular orbital energy levels. The low-lying LUMO levels of these materials afford them great potential as n-type organic semiconductors.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...