Understanding the reaction mechanisms of Pd-catalysed oxidation of alcohols and domino oxidation–arylation reactions using phenyl chloride as an oxidant†
Abstract
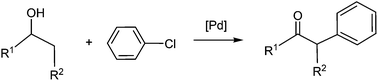
Density functional theory calculations were carried out to study the Pd-catalysed oxidation reactions of alcohols using phenyl chloride as an oxidant. Our calculations supported that the mechanism mainly involves oxidative addition, β-hydride elimination, reductive elimination, and finally ligand substitution. Through our calculations, we have explained why oxidation of secondary alcohol was experimentally observed but not that of primary alcohol. The oxidation products (ketones) of secondary alcohols bind much more weakly than the oxidation products (aldehydes) of primary alcohols, contributing to the reactivity difference. The mechanism of the Pd-catalysed domino oxidation–arylation reactions of secondary alcohols was also studied. We have explained the experimental observation that α-arylation of the oxidation products (ketones) of secondary alcohols occurs only when the temperature was raised to 80 °C from below 40 °C.

 Please wait while we load your content...
Please wait while we load your content...