Computation of standard equilibrium acidity of C–H acids in ionic media: shedding light on predicting changes of chemical behavior by switching solvent system from molecular to ionic†
Abstract
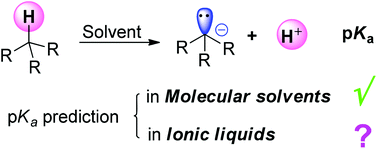
An SMD implicit–explicit approach was developed that allows reproduction of experimental pKa values of various carbon acids in ionic liquids with a mean unsigned error of 1.28 pK units, which is comparable in magnitude to those authentic pKa calculations in conventional molecular solvents. This model may be used for predicting more pKa values in RTILs and may also offer clues for studying other thermodynamic quantities.

 Please wait while we load your content...
Please wait while we load your content...