Asymmetric hydrogenation of α-arylacrylic and β-arylbut-3-enoic acids catalyzed by a Rh(i) complex of a monodentate secondary phosphine oxide ligand†
Abstract
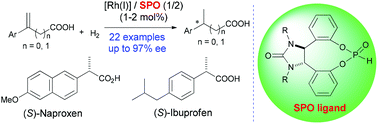
The RhI complexes of chiral secondary phosphine oxide ligands have been disclosed to be efficient for the catalytic asymmetric hydrogenation of α-arylacrylic and β-arylbut-3-enoic acids, providing the corresponding chiral α-arylpropanoic and β-arylbutanoic acids in excellent yields with up to 97% ee, including several anti-inflammatory drugs.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...