Asymmetric total synthesis of (−)-cebulactam A1†
Abstract
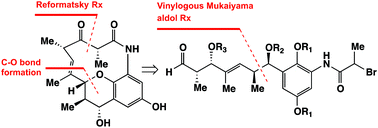
The total synthesis of (−)-cebulactam A1 (3) has been achieved for the first time in 18 steps. The key steps in this synthesis included an asymmetric chelation-controlled vinylogous Mukaiyama aldol reaction for the stereoselective synthesis of the stereogenic centers at the C8 and C9 positions, an intramolecular SmI2-mediated Reformatsky reaction for the formation of a macrocyclic lactam, and an SN2′ reaction for the stereoselective formation of the (E)-double bond linked tetrahydropyran moiety of cebulactam A1 (3).

 Please wait while we load your content...
Please wait while we load your content...