Supramolecular aggregation properties of 4-(1-morpholino)-1,8-naphthalimide based fluorescent materials†
Abstract
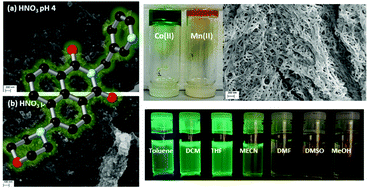
Here we report the synthesis of two morpholino-substituted naphthalimide ligands, N-(3-picolyl)-4-(1-morpholino)-1,8-naphthalimide L1 and N-benzyl-4-(1-morpholino)-1,8-naphthalimide L2, and study their supramolecular properties in the crystalline, solution and gel phases. These ligands were designed through incorporation of the morpholino group to enhance their photophysical and pH-responsive properties following recently reported N-(3-picolyl) naphthalimide metallogels. L1 was found to form metallogels on reaction with either Mn2+ or Co2+. The gels were found to be thermally and chemically responsive to various stimuli including pH. Conversely, L2 showed no reaction or coordination with transition metals, and did not gel under analogous conditions to L1. In the solution state, the fluorescence of both L1 and L2 exhibited pH responsiveness and counterion-influenced aggregation. The microparticle formation over the pH range was further investigated through Dynamic Light Scattering and Scanning Electron Microscopy. These two ligands illustrate how a modular ligand family can derive structure–function relationships and allow for systematic tuning, thus allowing for the future development of luminescent pH responsive soft materials.


 Please wait while we load your content...
Please wait while we load your content...