Fluorinated heptacyclic carbazole-based ladder-type acceptors with aliphatic side chains for efficient fullerene-free organic solar cells†
Abstract
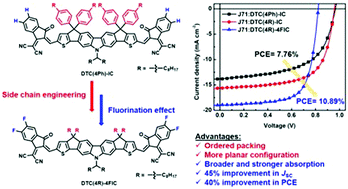
Molecular engineering of non-fullerene acceptors (NFAs) is a promising strategy to uncover new structure–property relationships and design principles for developing next-generation high performance n-type materials. Two dithienocyclopentacarbazole (DTC)-based NFAs, DTC(4R)-IC and DTC(4R)-4FIC, are synthesized to elucidate the effects of incorporating aliphatic side chains and 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (2FIC) end groups on the thermal and optoelectronic properties of these NFAs. An inverted organic solar cell architecture of ITO/ZnO/J71:NFA/MoO3/Ag is employed. By replacing the phenyl-based side chains with aliphatic side chains on the carbon bridges of the carbazole-based core, the device containing DTC(4R)-IC exhibits an enhanced PCE of 9.61%, a VOC of 0.94 V, a JSC of 16.44 mA cm−2, and a FF of 62.00%, whereas the device containing DTC(4Ph)-IC exhibits a PCE of 7.76%. The incorporation of 2FIC end groups affords DTC(4R)-4FIC featuring better device performance relative to DTC(4R)-IC, a more red-shifted absorption edge, and a broader absorption range upon fluorination. Notably, the device fabricated with DTC(4R)-4FIC affords a Voc of 0.82 V, a Jsc of 18.92 mA cm−2, a FF of 70.22% and a highest PCE of 10.89%, which is by far the highest PCE for NFA containing heptacyclic carbazole cores.


 Please wait while we load your content...
Please wait while we load your content...