In situ alternative switching between Ti4+ and Ti3+ driven by H2O2 in TiO2 nanostructures: mechanism of pseudo-Fenton reaction†
Abstract
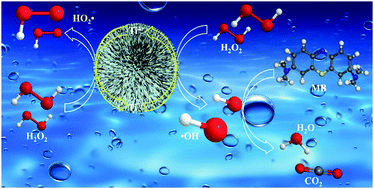
Here, we report the discovery that Ti4+ in TiO2 nanostructures can be reduced in situ to Ti3+ in the presence of H2O2 to generate hydroxyl radicals for the oxidation of organic pollutants. The Ti4+ in the TiO2 nanostructure and the reduced Ti3+ driven by H2O2 can alternatively switch in situ, which endows the catalyst with a long life and excellent cyclic ability.


 Please wait while we load your content...
Please wait while we load your content...