Planarity of N-aryl in appended 1,2,4-triazole-based o-carboranyl luminophores: a key factor to control intramolecular charge transfer†
Abstract
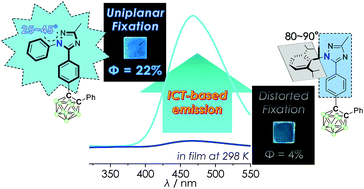
Two o-carboranyl compounds containing 1,2,4-triazole groups, each appended with an N-aryl ring (either N-phenyl or N-diisopropylphenyl), were strategically designed and prepared to elucidate the relationship between their structural orientation (planarity) and intramolecular charge transfer (ICT)-based radiative decay. The photophysical analysis of both compounds shows that the N-phenyl appended compound shows a much higher quantum yield and larger radiative decay constant for ICT-based emission in the rigid state (solution at 77 K and film) than those of the N-diisopropylphenyl appended compound, even exhibiting an additional ICT-based emission in solution at 298 K. Theoretical calculations on their ground and excited states indicate that the planarity of the N-aryl appended triazole moiety affects the efficiency of radiative decay for the ICT transition. These findings firmly establish a strong relationship between the planarity of the appended aryl groups relative to the triazole ring and ICT-based radiative decay in these o-carboranyl luminophores.


 Please wait while we load your content...
Please wait while we load your content...