Tuning the packing, interpenetration, and porosity of two-dimensional networks by metal ions and ligand side groups†
Abstract
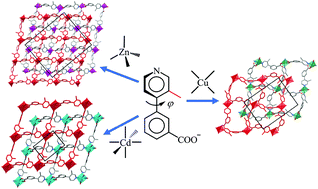
Solvothermal reactions of 3-(3-methylpyridin-4-yl)benzoic acid (Hmpba) with Zn(II), Cd(II), or Cu(II) salts yielded crystals of coordination polymers [Zn(mpba)2] (1), [Cd(mpba)2] (2), and [Cu(mpba)2]·guest (3·g), respectively, possessing two-dimensional (2D) 4-connected sql coordination networks with different packing/interpenetration modes. 1 and 2 both possess close-packing two-fold interpenetrated sql nets, but the ligand orientations in their single layers and the stacking manners of their double layers are quite different and have an inversed trend of complexity. The sql nets in 3 are non-interpenetrated, which loosely pack to give a porous structure with 1D channels. Interestingly, 1–3 have a completely inversed trend in packing/interpenetration and porosity, compared to the coordination networks constructed by the same metal ions and the ligand without the methyl group (3-(pyridin-4-yl)benzoic acid). The porous framework of 3 can be retained after guest removal, as demonstrated by the CO2 adsorption isotherms and high catalytic performances for cycloaddition of epoxides and CO2.


 Please wait while we load your content...
Please wait while we load your content...