Full water splitting by a nanoporous CeO2 nanowire array under alkaline conditions†
Abstract
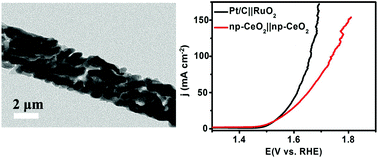
The most abundant rare earth metals in the Earth's crust have received considerable recent attention as efficient electrocatalysts for full water splitting, but it is highly desired to explore a new strategy to improve their catalytic activity. In this communication, we report the development of nanoporous CeO2 nanowire array on Ti mesh (np-CeO2/TM) derived from MnO2–CeO2/TM via an acid etching strategy, and MnO2 acts as a pore-forming agent through selective etching with oxalic acid. As a rare earth metal catalyst, np-CeO2/TM needs an overpotential of 91 mV for the hydrogen evolution reaction (HER) and 279 mV for the oxygen evolution reaction (OER) to drive a current density of 10 mV cm−2 in 1.0 M KOH, 93 mV and 101 mV less than that needed by MnO2–CeO2/TM, respectively. We also demonstrate the use of np-CeO2/TM to make a two-electrode electrolyzer capable of driving 10 mV cm−2 at a cell voltage of 1.57 V.


 Please wait while we load your content...
Please wait while we load your content...