Exploiting cation aggregation in new magnesium amidohaloaluminate electrolytes for magnesium batteries†
Abstract
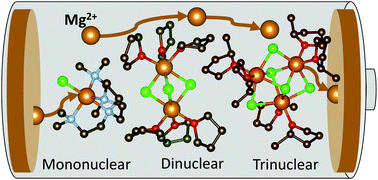
Mg batteries present an attractive and sustainable alternative to Li-ion batteries, wherein magnesium metal as an anode displays a superior theoretical volumetric energy density of 3833 A h L−1versus 2062 A h L−1 for lithium. An outstanding crucial bottleneck in realising their more widespread uptake is the development of suitable electrolytes, where electrode passivation, a limited electrochemical window, conditioning requirements, low ion mobility and low coulombic efficiencies all contribute to current limitations in Mg batteries. In an area thus far dominated by the thermodynamically stable [Mg2Cl3]+ dinuclear cation, we present here a novel family of magnesium amidohaloaluminate electrolytes [(Dipp)(SiMe3)2NAlCl3]− [MgxCl2x−1]+ where the magnesium chloride cation aggregation has been tailored (x = 1, 2, 3) by substitution of the coordinating ligand to the Mg2+ centre, and show how directly altering this cation affects battery performance (Dipp = 2,6-diisopropylphenyl, Me = methyl). The electrochemical activity of these new electrolytes has been evaluated by cyclic voltammetry, galvanostatic cycling and impedance spectroscopy in Mg–metal symmetrical cells as well as in battery cells with the Mo6S8 Chevrel phase cathode material against magnesium metal. The mononuclear and dinuclear magnesium amidohaloaluminate electrolytes facilitate reversible Mg plating and stripping from the Mg–metal anode with excellent stability, withstanding over 70 hours of continuous cycling. We demonstrate the compatibility of these novel electrolytes with the Mo6S8 Chevrel intercalation cathode material, allowing cycling of a Mg–metal cell up to 100 cycles with coulombic efficiencies above 95%.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...