Hexafluorosilicate anion in the formation of a coordination cage: anion competition†
Abstract
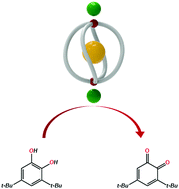
The construction of a coordination cage in a system of dual anions including hexafluorosilicate was investigated. The self-assembly of Cu(BF4)2 with L in the presence of (NH4)2SiF6 gives rise to a cage of [(SiF6)@Cu2L4](BF4)2, whereas the reaction of CuX2 (X− = NO3− and ClO4−) with L in the presence of (NH4)2SiF6 yields a cage of [(SiF6)@Cu2L4](SiF6). The anion exchange of [(SiF6)@Cu2L4](BF4)2 with NaPF6 in acetone gives rise to a 2D structure of [Cu(PF6)2L2]·C3H6O. Significant catalytic effects on catechol oxidation catalysis in dichloromethane were observed in the order [(SiF6)@Cu2L4](BF4)2·MeOH > [(SiF6)@Cu2L4](SiF6)·6MeOH ≫ [Cu(PF6)2L2]·C3H6O > Cu(BF4)2. The oxidation catalysis reactions using the coordination cages were efficiently completed within 20 min at 40 °C, the performance of which ranks among the most efficient yet recorded.


 Please wait while we load your content...
Please wait while we load your content...