Regulating the single-molecule magnetic properties of phenol oxygen-bridged binuclear lanthanide complexes through the electronic and spatial effect of the substituents†
Abstract
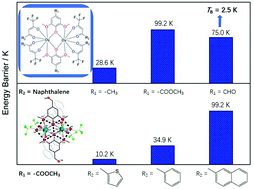
A series of binuclear lanthanide complexes with formulas [Dy2(DMOMP)2(TFNB)4]·CH3CH2OCH2CH3 (1), [Dy2(DMOEP)2(TFNB)4] (2), [Dy2(DMOEP)2(BTFA)4] (3), [Dy2(DMOEP)2(TTA)4] (4), and [Dy2(DMOAP)2(TFNB)4] (5), (DMOMP = 2,6-dimethoxy-4-methylphenol, DMOEP = methyl 3,5-dimethoxy-4-hydroxybenzoate, DMOAP = 3,5-dimethoxy-4-hydroxybenzaldehyde, TFNB = 4,4,4-trifluoro-1-(2-naphthyl)-1,3-butanedione, BTFA = benzoyltrifluoroacetone, TTA = 2-thenoyltrifluoroacetone) were structurally and magnetically characterized. The core structures of these complexes consist of dinuclear lanthanide(III) ions bridged firmly by phenoxyl O atoms from two nearly horizontal and isolated 2,6-dimethoxyphenol (DMOP) derivatives, which provide an ideal model to investigate the synergistic contribution of single-ion anisotropy and magnetic exchange interaction. In this case, the modification was carried out on the para position of the phenol hydroxyl of the DMOP bridge and on one side of the terminal substituent of the β-diketonate co-ligand with electron-donating/withdrawing substituents that also exhibit different steric hindrance. Following this, the effective energy barrier (Ueff) of magnetization reversal was enhanced by ten times in magnitude at the most, and the hysteresis temperature increased from zero to 2.5 K.


 Please wait while we load your content...
Please wait while we load your content...