Tetranuclear rare-earth complexes: energy barrier enhancement and two-step slow magnetic relaxation activated by ligand substitution†
Abstract
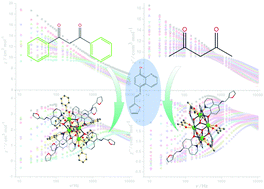
The reaction of 5-(2-furanimino)-8-hydroxylquinoline (HL) and RE(dbm)3·2H2O (Hdbm = 1,3-diphenyl-1,3-propanedione) afforded [RE4(dbm)4(L)6(μ3-OH)2] (RE = Y (1), Dy (2)), while under the same reaction conditions, but with the replacement of the co-ligand, another series of tetranuclear complexes, [RE4(acac)4(L)6(μ3-OH)2]·(CH3CN)n (n = 2 for RE = Y (3), Dy (4), Ho (5); n = 0 for RE = Tb (6); Hacac = acetylacetone), were obtained. Alternating current susceptibility measurements reveal that 2 displays single-molecule-magnet behavior with an effective energy barrier of Ueff = 117.4 K (τ0 = 1.79 × 10−8 s) under a zero direct-current field, while 4 presents two distinct slow magnetic relaxation processes with effective energy barriers of Ueff = 162.1 K for the fast relaxation (FR) process (τ0 = 8.53 × 10−11 s) and Ueff = 165.3 K for the slow relaxation (SR) process (τ0 = 1.14 × 10−9 s), which demonstrates that subtle changes of the coordination environment can drastically influence the overall magnetic properties.


 Please wait while we load your content...
Please wait while we load your content...