Base induced C–CN bond cleavage at room temperature: a convenient method for the activation of acetonitrile†
Abstract
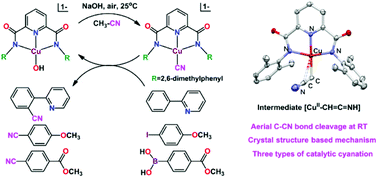
An efficient and simple method was explored for the C–CN cleavage of acetonitrile in air at room temperature. The activation reaction was mediated by the mononuclear complex of [MII(PyN2Ph2Me2)(OH)]1− (M = Cu, Ni; PyN2Ph2Me2 = N,N′-bis(2,6-dimethylphenyl)-2,6-pyridinedicarboxamidate(2-)) in the presence of NaOH. Acetonitrile splits into cyanide and formaldehyde via the intermediate η2-enaminemethide isomer species of [MII–(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)] and [MII–(N
NH)] and [MII–(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)]. A base-dioxygen induced reaction mechanism was proposed based on the crystal structures of intermediate complexes and organic adducts, which were characterized by X-ray crystallography, EPR spectroscopy, GC-MS techniques and DFT calculations. The nitrile-activated product [CuII–CN] was utilized as a cyanide source for the catalytic cyanation of iodobenzene, phenylboronic acid and 2-phenylpyridine.
CH2)]. A base-dioxygen induced reaction mechanism was proposed based on the crystal structures of intermediate complexes and organic adducts, which were characterized by X-ray crystallography, EPR spectroscopy, GC-MS techniques and DFT calculations. The nitrile-activated product [CuII–CN] was utilized as a cyanide source for the catalytic cyanation of iodobenzene, phenylboronic acid and 2-phenylpyridine.


 Please wait while we load your content...
Please wait while we load your content...