Room-temperature hydrogen generation from water and nanoscale Fe catalyzed by Pd†
Abstract
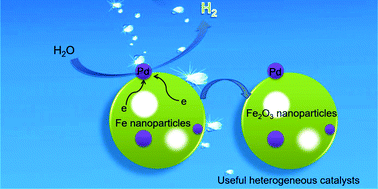
Hydrogen generation under mild conditions is a promising strategy and longstanding challenge for the development of novel clean energy resources. Most current systems involve organic/inorganic hydrides that are catalyzed in basic media and have to release the carbon oxides (CO or CO2). Herein, we describe an alternative pathway for highly efficient production of pure H2 (0.073 mmol gFe−1 min−1) through a redox reaction between water and Fe nanoparticles at room temperature catalyzed by Pd without the activation of light or electricity. Experimental results prove that the small size of Fe nanoparticles and the catalysis of Pd supported on its surface play a key role in the successful generation of hydrogen. With the catalysis of Pd, the reaction activation energy can be obviously reduced, which is beneficial for the enhanced hydrogen generation rate. The hydrogen can also be successfully released from the water and Fe nanoparticle system even at 0 °C reaction temperature, indicating its broad scope of application. Meanwhile, the remnants of the hydrogen generation system are Pd/Fe2O3 nanoparticles, which are useful and recyclable heterogeneous catalysts for various organic reactions.


 Please wait while we load your content...
Please wait while we load your content...