Ambient electrochemical N2-to-NH3 fixation enabled by Nb2O5 nanowire array†
Abstract
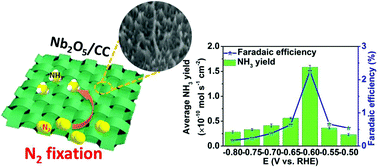
Electrochemical reduction offers a carbon-neutral process for energy-saving NH3 synthesis under ambient conditions, but this process involves difficulty in breaking the strong N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of inert N2 and the challenge of N2 activation, underlining the need for high-performance electrocatalysts for the nitrogen reduction reaction (NRR). Herein, we report our recent effort in this direction by in situ hydrothermally growing a Nb2O5 nanowire array on carbon cloth (Nb2O5/CC) as a self-standing NRR catalyst electrode toward artificial N2 fixation with high selectivity under ambient conditions. In 0.1 M Na2SO4, such Nb2O5/CC achieves an NH3 formation rate of 1.58 × 10−10 mol s−1 cm−2 and a high faradaic efficiency of 2.26% at −0.60 V, rivaling the performances of most reported non-noble metal NRR electrocatalysts in neutral medium. Notably, this catalyst also shows high electrochemical stability during electrolysis and recycling tests. Density functional theory calculations reveal that a distal associative route is involved in the reaction pathways during the NRR over the Nb2O5 (001) facet. This work would open up an exciting new avenue to explore the design of self-standing 3D electrodes made of transition metal oxides for the NRR and other applications.
N bond of inert N2 and the challenge of N2 activation, underlining the need for high-performance electrocatalysts for the nitrogen reduction reaction (NRR). Herein, we report our recent effort in this direction by in situ hydrothermally growing a Nb2O5 nanowire array on carbon cloth (Nb2O5/CC) as a self-standing NRR catalyst electrode toward artificial N2 fixation with high selectivity under ambient conditions. In 0.1 M Na2SO4, such Nb2O5/CC achieves an NH3 formation rate of 1.58 × 10−10 mol s−1 cm−2 and a high faradaic efficiency of 2.26% at −0.60 V, rivaling the performances of most reported non-noble metal NRR electrocatalysts in neutral medium. Notably, this catalyst also shows high electrochemical stability during electrolysis and recycling tests. Density functional theory calculations reveal that a distal associative route is involved in the reaction pathways during the NRR over the Nb2O5 (001) facet. This work would open up an exciting new avenue to explore the design of self-standing 3D electrodes made of transition metal oxides for the NRR and other applications.


 Please wait while we load your content...
Please wait while we load your content...