Sn(TFSI)2 as a suitable salt for the electrodeposition of nanostructured Cu6Sn5–Sn composites obtained on a Cu electrode in an ionic liquid†
Abstract
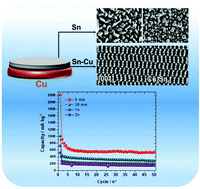
The preparation of binder and carbon-free electrodes is of great interest owing to their higher energy density. In this scope, electrodeposition is a suitable method that can be easily scaled up provided that suitable chemical reactants are used. In this work, we used a highly soluble Sn precursor based on TFSI counter ions dissolved in an [EMIm+][TFSI−] ionic liquid. The use of similar anionic groups in both the Sn precursor and the solvent allowed avoiding impurities typically encountered when using the Sn chloride precursor. The resulting solution was characterized by cyclic voltammetry using either an inert (Mo) or a reactive substrate (Cu). In both cases, the electrodeposition occurred in a diffusion controlled process. In the case of Cu, however, a Cu–Sn alloy that is the η-Cu6Sn5 phase was identified. An FIB cross section revealed that Cu and Sn interdiffused and that no epitaxial growth occurred. Prolonging the electrodeposition time favored the formation of β-Sn because Cu could no longer diffuse within the deposited structure. This resulted in a mixed β-Sn/η-Cu6Sn5 composite whose proportions depended on the deposition duration time. The deposited samples were directly assembled in lithium coin cells to characterize the capacity and cyclability of the binder and carbon-free electrodes. Increasing the mass of the deposited sample degraded the capacity and reversibility of the system which was explained by a lower ability to accommodate the volume variation occurring during the electrochemical lithiation/delithiation process.


 Please wait while we load your content...
Please wait while we load your content...