Macroscopic and microscopic investigation of uranium elimination by Ca–Mg–Al-layered double hydroxide supported nanoscale zero valent iron†
Abstract
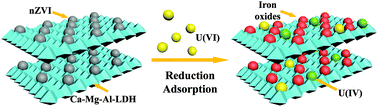
In the past few decades, uranium (235, 238U(VI)) has turned into a forefront environmental issue due to its extensive use in the nuclear industry and its toxicity and radioactivity. Herein, nanoscale zero valent iron (nZVI), which was supported on Ca–Mg–Al-layered double hydroxide (Ca–Mg–Al-LDH/nZVI), was fabricated via an in situ growth route and employed for U(VI) decontamination from aqueous solutions. Spectroscopy and microscopy (SEM, TEM, FTIR, XRD, BET, and XPS) technologies were applied for the investigation of the properties of Ca–Mg–Al-LDH/nZVI and the U(VI) elimination mechanism. Ca–Mg–Al-LDH/nZVI had a remarkable BET surface (426.8 m2 g−1), abundant functional groups (i.e., Fe–O, Al–O, –OH, etc.), high efficiency (4 h to achieve equilibrium) and large adsorption capacities (Qmax = 216.1 mg g−1) for U(VI) removal. The decontamination process of U(VI) was observed to be pH-dependent and ionic strength-independent, suggesting that the adsorption was predominated by inner-sphere surface coordination. XPS spectroscopy analyses indicated that the reduction and adsorption of nZVI and the adsorption of Ca–Mg–Al-LDH dominated U(VI) elimination on Ca–Mg–Al-LDH/nZVI. The environmentally friendly synthesis method, excellent physicochemical properties and remarkable removal performance suggested that Ca–Mg–Al-LDH/nZVI was applicable as a potential adsorbent for U(VI) decontamination from wastewater in environmental pollution remediation.


 Please wait while we load your content...
Please wait while we load your content...