Cube-like CuCoO nanostructures on reduced graphene oxide for H2 generation from ammonia borane†
Abstract
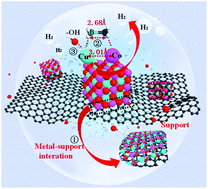
Ammonia borane (AB) is an excellent hydrogen-storage material for fuel cells. However, the release of H2 from AB is limited by using expensive noble-metal-based catalysts. Here, we build up a cube-like Cu0.5Co0.5O nanostructure on reduced graphene oxide (rGO) for the high-efficiency hydrolysis of AB. The Cu0.5Co0.5O-rGO catalyst can achieve a high total turnover frequency of 81.7 (H2) mol·(Cat-metal)mol−1·min−1, which is one of the best values ever reported for noble metal-free catalysts. The catalyst also shows good stability by maintaining 88.3% activity after 5 runs. Synchrotron radiation-based in situ X-ray absorption spectroscopy is used to probe the catalytic mechanism, which reveals that the Cu sites in the catalyst can activate water and then collaborate with Co to anchor the AB molecules for high catalytic activity. The high performance of Cu0.5Co0.5O-rGO can be attributed to both the coordination of Cu and Co in the cube-like structure and the metal-support interaction. The deactivation of Cu0.5Co0.5O-rGO after 10 cycles is also studied, which can be attributed to the loss of cube-like structure and the reduction of Cu and Co. The in-depth understanding of the catalytic process may significantly pave the way for the rational design of highly efficient catalysts.


 Please wait while we load your content...
Please wait while we load your content...