Selective high adsorption capacity for Congo red dye of a new 3D supramolecular complex and its magnetic hybrid†
Abstract
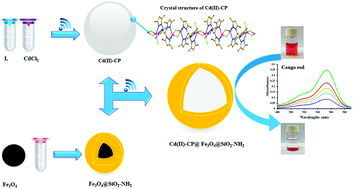
A new non-covalently bonded 3D supramolecular architecture, namely, [CdII2Cl2(μ-Cl)2(μ-L)]n [where L = 1,1,3,3-tetrakis(3,5-dimethyl-1-pyrazolyl)propane] was synthesized and structurally characterized. In this structure, the N4-coordinated pyrazole-based ligand displayed a chelating–bridging mode and bounds to two neighboring CdII centers to create a 1D sinusoidal chain along the b-axis. Each of the cadmium(II) sites adopted a distorted square pyramidal arrangement with a CdN2Cl3 core. The chains were further stabilized by non-covalent bonds such as C–H⋯Cl and C–H⋯π to generate a 3D network. This structure has the ability to incorporate Fe3O4@SiO2-NH2 nanoparticles and build up an Fe3O4@SiO2-NH2@Cd(II)-CP hybrid using a sonochemical method. The bonding established between the Cd(II)-CP and amino-functionalized Fe3O4 nanoparticles improved the separation of the CP by magnetic decantation. The products exhibited a high capacity and high selectivity to adsorb Congo red (CR) from an aqueous solution. The sorption kinetics and equilibrium isotherm were studied in detail using four kinetics and three isotherm models. According to the results that were obtained, the mechanism of the sorption process followed the pseudo-second-order kinetic and Langmuir isotherm models. In general, the magnetic hybrid under consideration can be proposed as a reversible catalyst for the selective adsorption of CR with facile separation from the environment using a magnetic bar.


 Please wait while we load your content...
Please wait while we load your content...